






We understand the highly regulated nature of the medical device industry and the critical role that translation quality plays in achieving regional and international success. Our experience coupled with certified processes that are also HIPAA compliant ensure we deliver quality while maintaining robust information and data security.



Translations done on client's Medical reports related to peripheral vascular disease and hypertension.


Translated client's equipment manual related to contouring in RT-Structure Sets and renaming partial structures according to TG263 standard.


Translated the production report for Upacicalcet sodium hydrate (AJT240 Na), an active pharmaceutical ingredient.


Translated and Transcribed, a clinical project where the client interviewed a set of patients about a particular health issue and their symptoms.


High-quality translations were completed on Sanofi's adverse event reports, developed by the Council for International Organizations of Medical Sciences (CIOMS).


Translated 121 files of Pharmaceutical and Chemical–Formulation Development and Quality Assurance–Standards and Quality Test Reports for Alvogen.


Translated catalogs for client's various medical solutions that help with treating and maintaining the skin.


Translated a Clinical Research Paper related to High-Intensity focused Ultrasound that Decreases Subcutaneous Fat Tissue Thickness by Increasing Apoptosis and Autophagy.





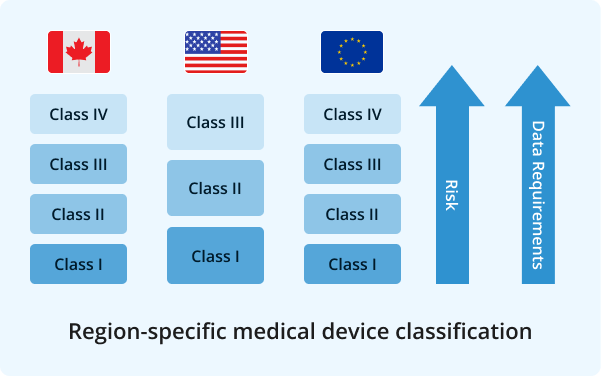
Based on your request, we make sure that your translations comply with global regulatory standards. Additionally, we can format your documents to meet the specific regulations set by different countries, including but not limited to:
We've designed our localization experience with certified processes and efficient technology, ensuring a seamless delivery of consistent quality that enhances client satisfaction.

Specialized Subject-
Area Matching

Quality-
Driven Technology

24x7 Dedicated
Localization Manager

ROI-Driven
Localization Solutions

In-House Expertise

ISO-Certified Processes
We assign translators that are in-field specialists in your specific domain and understand the intricacies of the target language. Each translator possesses a Ph.D. or Master's degree in the relevant subject, along with years of extensive translation experience. Our rigorous standards contribute to the superior quality of your translated outcomes.
We've helped many medical clients with the best quality medical translation services. Here's what they have to say about the quality and value we provide.

I have been using Ulatus services regularly for some time now and their quality of work is really good. The style and accuracy with which they translated my various documents was exactly what I expected it to be. I am really impressed with their team who worked closely with me to translate our vision into reality.

Through their excellent project management system, Ulatus surpassed all my expectations and passed fairly. Given the demands of this project, it was difficult to meet my requirements but Ulatus successfully delivered my work following the timelines and high-quality standards. It is a miracle company.

I am grateful to the entire team for being so generous and sincere towards our requirements. At this point, we can almost blindly trust you with everything. The team barely needed our supervision and the result delivered was exactly what we were looking for. Honestly, what else could we ask for! Thank you, Ulatus. You’re simply the best!
Ulatus is trusted by over 200,000 clients worldwide for their medical translation needs, boasting an exceptional client satisfaction rate of 99.45%.