Crimson Interactive, the parent company that governs Ulatus operations, is certified by the International Organization for Standardization (ISO) for having in place robust information security and quality management systems.
The ISO is a network of national standards institutes of 162 countries with a central authority in Geneva, Switzerland. The organization has 3,368 technical bodies that develop and monitor standards. It sets standards for companies to follow, and it certifies them after a successful audit of their systems and processes.
We consider all four certifications critical to demonstrating our commitment to the expectations of our clients and business partners worldwide. They enable us to deliver secure, accurate, and timely assignments, on every occasion for every client.




We find the best people and empower them with the best technology assistance. Translation is not a transaction. Clients entrust their intellectual labors to us, the fruits of years of work, research, development, and the distillation of experience. There’s no greater trust than this and we have to constantly strive to requite their faith. This certification is a testimony to our dedication to providing the best service that all our clients deserve. At the end of the day, providing language solutions is about people. Our 99.45% client satisfaction rate demonstrates how much we’ve achieved, and how hard we have to work to maintain this exceptionally high standard.

CEO
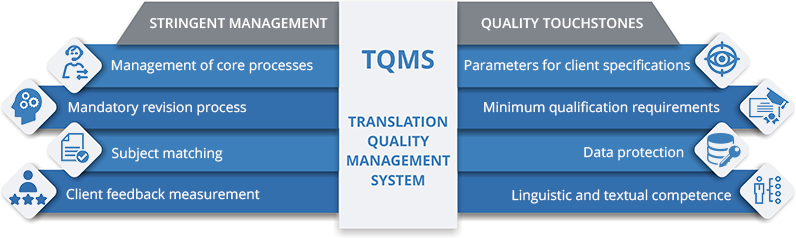
Ulatus, one of the leading language solution providers globally, is now among the 1% of translation companies in the world to be ISO 17100:2015 certified. This certification is only accredited to translation companies adhering to the stringent requirements essential for the delivery of quality translation, every single time.
In order to be ISO 17100:2015 certified, organizations must maintain the highest standards in all aspects pertaining to the quality and delivery of translation solutions. It includes provisions for translation service providers (TSPs) concerning the management of core processes, minimum qualification requirements, the availability and management of resources, and other necessary actions. Not only did Ulatus satisfy those competency benchmarks, it led the industry by demonstrating its prowess in research, subject area matching, and information acquisition and processing.
Just as our clients place a high value on confidentiality, we place a high value on earning their trust. To this end, we’ve invested huge efforts into ensuring that our information security system meets the highest standards in the industry.

CEO
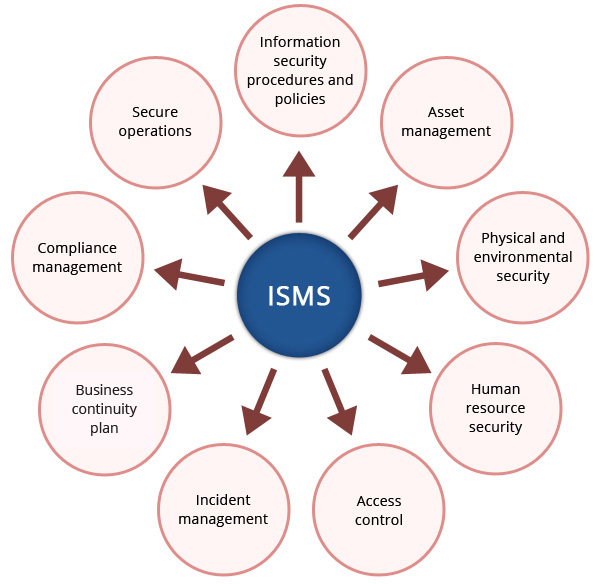
At Crimson Interactive, the parent company of Ulatus, we adhere to the most stringent standards of information security and client confidentiality. We are proud to be acknowledged by the internationally renowned ISO/IEC 27001:2022 certification for having established a robust Information Security Management System (ISMS) that ensures your trust.
This certification governs the management of information security in processes related to Information Technology, Client Servicing, Operations, Human Resources, and Administration. It is a testament to the fact we understand that your personal information, your research, and manuscripts are among your most important assets. We will always treat them with the greatest integrity.
Innovation is one of our key strengths, and we’ve designed a quality management system that demonstrates our commitment to delivering accuracy and accountability. ISO certification is a very important milestone for us, but it is only one cog in the wheel. We’ve met the standards, and our constant mission is to surpass them.

CEO
At Crimson Interactive, the parent company of Ulatus, we adhere to the highest standards of accuracy and reliability. We are proud to be certified by the internationally renowned ISO 9001:2015 in recognition for our robust Quality Management System (QMS) that delivers the highest level of quality in the industry.
This certification specifically demonstrates that an organization has proven not only its ability to consistently provide products that meet applicable statutory and regulatory requirements but that the system itself undergoes continual improvements. At Ulatus, we aim to deliver both products and processes that enhance customer satisfaction. We consistently endeavor to deliver superior translations, and we are constantly improving and streamlining our processes. We ask our clients for feedback on a regular basis, and we take it very seriously. In addition, we make constant efforts to educate and train our proofreaders and translators at all times.
Ulatus has been involved in numerous projects related to the medical industry. Our effort to serve the pharmaceutical and the life science industry with our language solutions has been met with an enviable list of happy clients. To further add credibility to our ability and experience in this regard we’ve also been “ISO 13485:2016 Medical devices — Quality management systems — Requirements for regulatory purposes” certified. This means our language solution meets up to the highest industry standards for pharma and life science related products and services.
ISO 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide medical devices and related services that consistently meet customer and applicable regulatory requirements. Such organizations can be involved in one or more stages of the life-cycle, including design and development, production, storage and distribution, installation, or servicing of a medical device and design and development or provision of associated activities (e.g. technical support). ISO 13485:2016 can also be used by suppliers or external parties that provide product, including quality management system-related services to such organizations.
ISO 13485 also reflects the increased regulatory requirements for organizations across the medical devices supply chain. We’re now one of the very few language solutions companies that possess our current combination of technology and quality certifications along with proven medical compliance standards.